April 25, 2023
LOGISTEED, Ltd. pleased to inform that the company newly introduced document record management system which supports GMP*¹ for our Group’s pharmaceutical logistics service.
Data integrity is required not only in the pharmaceutical manufacturing process but also in the distribution process to prevent the risk of quality loss and ensure patient safety. By considering that the Ministry of Health, Labor and Welfare might enact a ministerial ordinance for the voluntary standards of GDP (Good Distribution Practices) in the future, we have introduced HITQUAA*², the document management system of Hitachi Industry & Control Solutions, Ltd., which has a proven track record with pharmaceutical manufacturers, etc. This is their first attempt to introduce it to logistics operators, and it will be a case of deploying similar level of high-quality document record management as in the manufacturing domain to the logistics domain. By introducing the system, it will solve the conflicting issues of strict management and streamlining of approval work, which are difficult with conventional management methods centered on hardcopy, and it will contribute to strengthening the safety and reliability of pharmaceutical distribution.
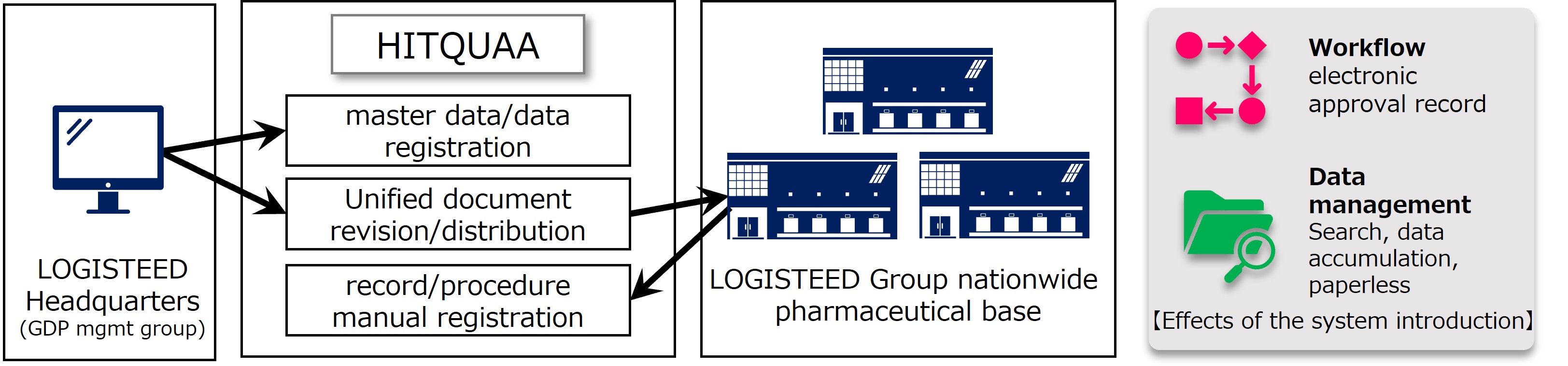 |
With this system, it realizes strict management that complies with the ER/ES*³ guidelines set by the Ministry of Health, Labor and Welfare, such as system control of user-operations, prevention of document loss due to tampering or erroneous operation, assurance of document originality, and electronic signature function, audit trail function for electronic records. In addition, DX reduces costs of paper and storage, and it enables efficient storage management that speeds up document search and approval work, which used to take time a lot.
From April 2023
Note
*1)GMP: Good Manufacturing Practice. It is pharmaceutical manufacturing control and quality control standards established by the Ministry of Health, Labor and Welfare.
*2) HITQUAA: A registered trademark of Hitachi Industry & Control Solutions, Ltd.
*3) ER/ES Guidelines: The Ministry of Health, Labor and Welfare stipulates necessary requirements for using electromagnetic records and signatures regarding source document or document related to drug approval and applications.
Our Service Portfolio